Atomic Structure
An atom is composed of three types of subatomic particles: the proton, neutron, and electron.
| Particle | Mass/g | Charge/$q_e$ |
|---|---|---|
| Proton | 1.6727 x 10-24 | +1 |
| Neutron | 1.6750 x 10-24 | 0 |
| Electron | 9.110 x 10-28 | -1 |
Here, $q_e$ is the elementary charge, a fundamental constant of nature, which is equal to $1.6021766208\times 10^{-19}$ coulombs.
Protrons and neutrons have similar masses and electrons are much lighter (over 1,000 times lighter).
Protons and electrons have equal and opposite charges while neutrons have no charge.
We have the following simple picture of the atom.
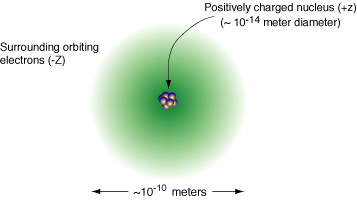
The atom is comprised of a positively charged nucleus composed of protons and neutrons. This small nucleus is surrounded by orbiting electrons. Because the protons and neutrons are so much more massive than the electrons, virtually all the mass of the atom is located in the nucleus. The light negatively charged electrons move around in an orbit in the space around the nucleus.
We use the following symbol to describe the atom:
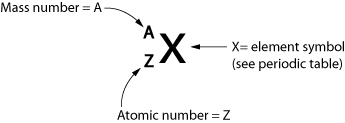
A= Z + N, where N is the number of neutrons.
If you add or subtract a proton from the nucleus, you create a new element.
If you add or subtract a neutron from the nucleus, you create a new isotope of the same element you started with.
In a neutral atom, the number of positively charged protons in the nucleus is equal to the number of orbiting electrons.
The Hydrogen Atom
Let's look at the simplest example of an atom, the hydrogen atom.
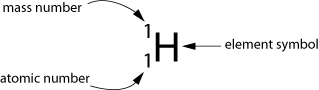
The atom consists of a proton and an electron held together by the electromagnetic force between the positively charged proton and the negatively charged electron.
The electron orbits around the proton because it is the lighter particle, sort of like the earth orbits around the sun, There are, however, big differences in the picture of the earth going around the sun and the electron going around the nucleus. This is because protons, neutrons and electrons exist on a length scale so small that quantum mechanics is required to understand the electron's orbit around the nucleus. We will learn more about the quantum theory of the atom later.
When we add neutrons to the nucleus of $^1_1$H we can make the isotopes of hydrogen. Here are three common isotopes of hydrogen.

If we add a proton to the hydrogen nucleus we would get helium (a different element). Here are two common isotopes of helium.

Another example is carbon.

Because the element symbol and atomic number are redundant, you will often see isotopes written without the atomic number. For example, you would see 12C only.
How many electrons, protons and neutrons are contained in the isotope $^{35}_{17}$Cl?
The number of protons is given by the atomic number, the bottom number, so the number of protons is 17. This is a neutral atom, so there will be an equal amount of negatively charged electrons to balance out the positively charged protons, thus making the number of electrons 17 also.
We know that the atomic mass is
where N is the number of neutrons. Rearranging the equation we get
Plugging in the numbers we already know, we get
Now you might think that an atomic nucleus with lots of protons (like 12C ) would fly apart from the electrical repulsions between positively charged protons. It turns out that these forces of electrical repulsions are overcome by an attractive force between protons and neutrons called the strong nuclear force. At small distances inside a nucleus, this force is stronger than the electromagnetic forces of repulsion, but at larger distances it becomes much weaker.
Atomic Mass
Grams is not a very convenient unit for atomic masses, so a new unit called the atomic mass unit (u) is defined.
1 u = 1.66053904 x 10 -24 g
Reexpressing the subatomic particle masses in terms of atomic mass units we have
| Particle | Mass/g | Mass/u |
|---|---|---|
| proton | 1.672621898 x 10-24 | 1.007276467284985 |
| neutron | 1.674927471 x 10-24 | 1.008664915821552 |
| electron | 9.10938356 x 10-28 | 0.0005485799093287202 |
Using an instrument called a mass spectrometer we can very accurately measure the mass of atoms and molecules. Here are some measured isotope masses using a mass spectrometer.
| Isotope | Mass/u |
|---|---|
| 2H | 2.01410177785 |
| 4He | 4.00260325415 |
| 8Be | 8.005305103 |
| 12C | 12.000000 |
| 16O | 15.99491461956 |
| 24Mg | 23.985041699 |
That 12C has a mass of exactly 12.000000 u is not a coincidence. A mass spectrometer can only measure mass differences accurately. To solve this problem the 12C isotope is defined to have a mass of exactly 12 u. Then everything else is measured relative to 12C.
As you might expect different isotopes of the same element will have different masses. If you look at the periodic table, however, you'll notice that there is only one number listed for the mass of each element. How can you only have one mass if there is more than one isotope of each element?
The answer is that the mass under each element is the weighted average of all of the isotope masses for that element. In this weighted average, the weights are the percent abundance that each isotope occurs in nature.
For example, if you analyzed a lump of pure carbon from the planet Earth, you would find that
98.89 % of all carbon atoms on earth are 12C atoms, and
1.11 % of all carbon atoms on earth are 13C atoms.
So the weighted average mass of carbon is
It is possible that on a planet in a far away galaxy, the natural abundances of carbon isotopes may be different, and therefore they would have slightly different numbers under carbon in their periodic table. The masses of the isotopes, however, are the same everywhere.
The natural abundance of 63Cu is 69.09 % and for 65Cu is 30.19 %. If the atomic weight of 63Cu is 62.93 u and 65Cu is 64.93 u, what is the average atomic weight for natural copper?

