Ionic Solids
When cations and anions precipitate out of a saturated solution they crystallize into a lattice arrangement that maximizes the attractive forces between cations and anions while minimizing the repulsive forces between ions of the same charge. For example, shown below is the arrangement of ions found in crystalline sodium chloride.
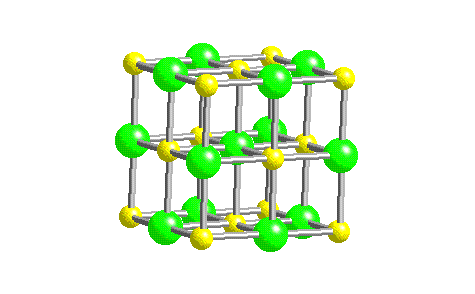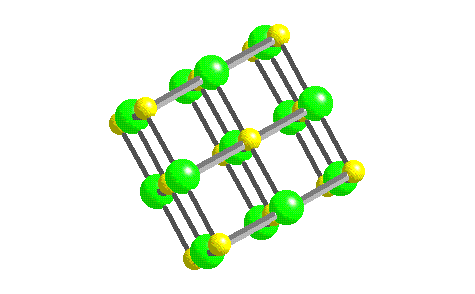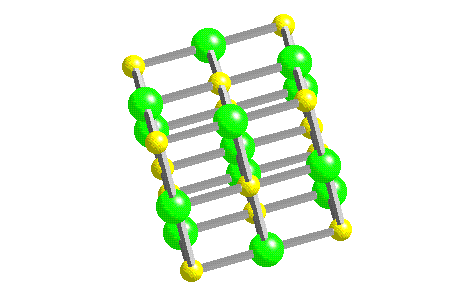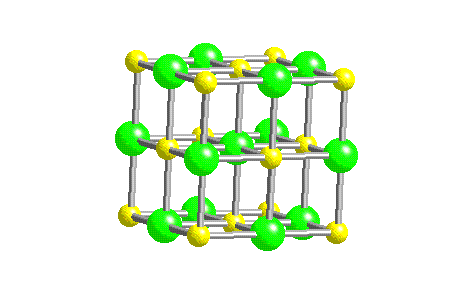
- Space Filling Model of NaCl Crystal Structure.
The chloride ions are represented as green, and the sodium ions as yellow in this figure. To better understand the energetics of this arrangement let's examine Coulomb's Law for the potential energy of interaction between two charges:

In NaCl we have three types of Coulombic interactions, an attractive interaction between sodium cations and chloride anions, and repulsive interactions between sodium cations and between chloride anions.
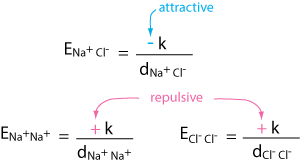
For a mole of solid NaCl, the sum of all these Coulombic energies for all cations and anions in a crystal is -861 kJ/mole.
| Na+(g) + Cl-(g)→NaCl(s) | ΔU = -861 kJ/mole |
Lattice Energy- change in energy when completely gaseous atoms are brought together into 1 mole of a solid ionic substance.
As we see from Coulomb's law, the lattice energy is related to the charge and distance between ions in the solid. Smaller the ions will have smaller distances between them in a solid, and thus have higher lattice energies.
Born-Haber Cycle
Lattice Energies can also be measured experimentally using Hess's law in what is called the Born-Haber Cycle. Recall that Hess's law tells us that the change in energy when going from reactant to products is the same whether the reaction takes place in one step or in a series of steps. For example, given the enthalpy changes for the following reactions we can obtain the enthalpy change for the formation of the crystalline NaCl lattice.
| Cl-(g) | → | Cl(g) + e- | ΔH = -E.A.(Cl) = 349 kJ/mole |
| Na+(g) + e- | → | Na(g) | ΔH = -E.A.(Na) = -496 kJ/mole |
| Na(g) | → | Na(s) | ΔH = -104.8 kJ/mole |
| Cl(g) | → | 1/2 Cl2(g) | ΔH = -120.5 kJ/mole |
| Na(g) + 1/2 Cl2(g) | → | NaCl(s) | ΔH = -411.2 kJ/mole |
| -------------------------------------------------------------------------------------------------------- | |||
Na+(g) + Cl-(g)
| → |
NaCl(s) |
ΔH = -783.5 kJ/mole |
|
That is pretty close to our calculated number of -861 kJ/mole (< 10% error). But, of course, our first calculation was based on infinitely seperated ions at 0 K, and the Born-Haber cycle is based on reaction enthalpy changes at room temperature and pressure, so we expect some differences.
Homework from Chemisty, The Central Science, 10th Ed.
8.13, 8.15, 8.17, 8.19, 8.21, 8.23, 8.25

