An important concept for understanding how heat is measured is the concept of heat capacity.
Heat Capacity: amount of heat needed to raise an object's temperature by 1 degree Kelvin.
More specifically we define
Molar Heat Capacity: amount of heat required to raise the temperature of one mole of a substance by one degree Kelvin.
Specific Heat Capacity: amount of heat required to raise the temperature of one gram of a substance by one degree Kelvin.
The heat capacity can vary quite a bit, particularly for gasses, depending on whether the pressure or volume is held constant as heat is added. Therefore, we further distinguish the heat capacities with the following symbols:
CV: Molar Heat Capacity at constant volume.
CP: Molar Heat Capacity at constant pressure.
CS: Specific Heat Capacity at constant pressure.
Since most processes discussed in this course are carried out at a constant 1 atmosphere of pressure, we will emphasize CS and CP.
If we have n moles of a substance, the heat transferred and the corresponding change in temperature are given by
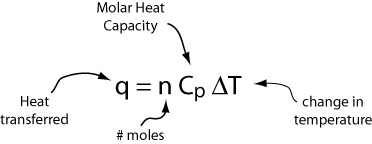
Alternatively, if we have m grams of a substance, the heat transferred and the corresponding change in temperature are given by
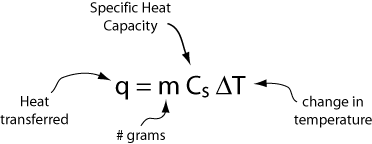
Below are the specific heats of some common substances
| Compound | Temperature (°C) | Specific Heat (J/g-°C) |
|---|---|---|
| H2O(l) | 15 | 4.1814 |
| H2O(s), ice | -11 | 2.03 |
| CaCO3(s) | 0 | 0.85 |
| MgO(s) | 0 | 0.87 |
| SiO2(s) | 25 | 0.739 |
| O2(g) | 25 | 0.917 |
The specific heat of water is one of the highest known specific heats. It is several times higher than that of compounds like limestone (CaCO3) and quartz (SiO2), which are the major constituents of rocks. Water can absorb or give up large amounts of heat with a smaller change in temperature than rocks (CaCO3, SiO2, MgO). That's why temperature fluctuations are smaller near large bodies of water like the ocean or Lake Erie than someplace like the desert in Arizona.
A piece of iron with a mass of 72.4 grams is heated to 100°C and plunged into 100 grams of water that is initially at 10.0°C. Calculate the final temperature that is reached assuming no heat loss to the surroundings. The heat capacities of iron and water are Cs(H2O) = 4.18 J/g-°C and Cs(Fe) = 0.449 J/g-°C.
First of all, we note that the heat gained by the cooler body + the heat lost by the warmer body = 0
In other words
qH2O = - qFe
thus we write
mH2O Cs(H2O) ΔTH2O = - mFe Cs(Fe) ΔTFe
at equilibrium we have
Tf(H2O) = Tf(Fe)
so we solve for Tf
mH2O Cs(H2O) [ Tf - Ti (H2O)] = - mFe Cs(Fe) [Tf - Ti(Fe)]
and obtain
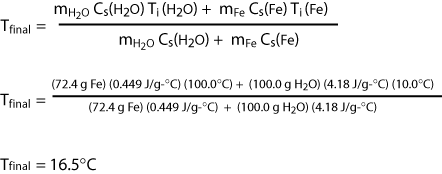
The heat flow associated with a chemical reaction is experimentally measured using a device called a calorimeter.
- Calorimetry:
- the science of measuring heat flow (based on observing the temperature change when a body absorbs or discharges heat).
Consider a reaction that gives off energy to its surroundings
A + B → C + D + Energy
We know the energy given off by this reaction will be transferred to the surroundings as heat and work.
ΔU= q + w
Now, from
Work = - p · ΔV
we know that if we seal the reaction in a vessel strong enough then that the volume can't change, and thus there can be no work done by the reaction since ΔV = 0. In this case we will have
ΔU=q at constant volume.
That is, all the energy will be transferred as heat into the surroundings. Therefore, if we surround our sealed chemical reaction container (i.e., our system) with substances of known heat capacity, then all we need to do is measure the temperature change of these surroundings to determine q (i.e., the heat evolved) and thus the total change in energy.
This is done in what is called a Bomb Calorimeter.
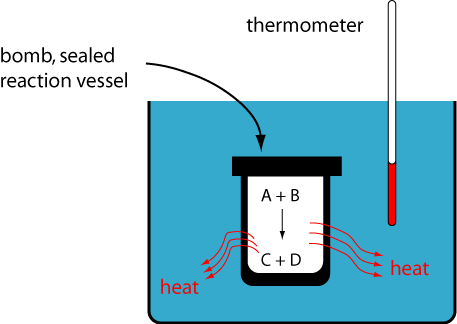
- Bomb Calorimeter:
- Calorimeter designed to measure heat flow with constant volume (no work is possible).
1.00 grams of N2H4, hydrazine, is burned in a bomb calorimeter
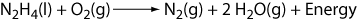
and the temperature of the calorimeter increases by 3.51°C. The bomb calorimeter has a heat capacity of 5.510 kJ/°C. What is the quantity of heat evolved?
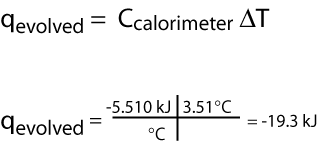
What is the heat evolved per mole of N2H4?

Thus, we can finally write
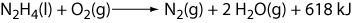
We have measured an energy change of 618 kJ for this reaction (i.e., the heat evolved at constant volume). Remember that ΔU is a state function (unlike q), so this number is good regardless of the history of the reactants and products. That is, 1 mole of hydrazine will always react with 1 mole oxygen and give 618 kJ of energy.
Note: These quizzes may use E, instead of U for the internal energy
Homework from Chemisty, The Central Science, 10th Ed.
5.27, 5.47, 5.49, 5.51

