Wave-Particle Duality of Light
Quantum theory tells us that both light and matter consists of tiny particles which have wavelike properties associated with them. Light is composed of particles called photons, and matter is composed of particles called electrons, protons, neutrons. It's only when the mass of a particle gets small enough that its wavelike properties show up.
To help understand all this let's look at how light behaves as a wave and as a particle.
Wave-like Behavior of Light
In the 1600s Christiaan Huygens, a Dutch physicist, showed that light behaves like a wave.

One behavior of waves is Diffraction

As the width of the slit becomes larger than the wavelength the wave is diffracted less.
Another behavior of waves is Interference

It was James Clerk Maxwell who showed in the 1800s that light is an electromagnetic wave that travels through space at the speed of light. The frequency of light is related to its wavelength according to
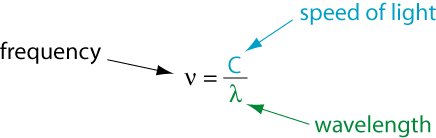
Let's look at an example calculation.
The light blue glow given off by mercury street lamps has a wavelength of λ = 436nm. What is its frequency?

The unit s-1 is so common when talking about waves that it was given the name Hertz. That is, 1 s-1 = 1 Hz. Thus, we would say that light with a wavelength of 436 nm corresponds to a frequency of 6.88 × 1014 Hertz.
The region from λ ≈ 400-750 nm is visible to the human eye and is therefore called the visible region of the electromagnetic radiation. As we saw in the example above, blue light is near the high frequency limit of our eyes. Red light, with wavelengths near 750 nm are at the low frequency limit of our eyes. Light that contains all frequencies in the visible region will appear as white light.
More generally, the different regions of the electromagnetic spectrum are given different names. Below are the names given to the different regions (frequency ranges) of light according to their frequency range.
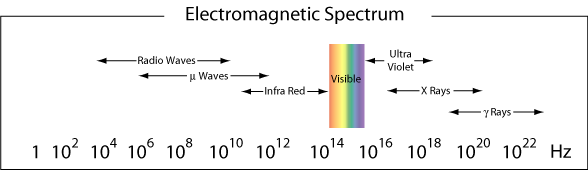
Particle-Like Behavior of Light
At this point you may think that it's pretty obvious that light behaves like a wave. So, why how do we know that light is really composed of particles called photons? Support for this idea comes from an experiment that is called the photoelectric effect.

An important feature of this experiment is that the electron is emitted from the metal with a specific kinetic energy (i.e. a specific speed).
Now anyone who is familiar with the behavior of waves knows that the energy associated with a wave is related to its amplitude or intensity. For example, at the ocean the bigger the wave, the higher the energy associated with the wave. It's not the small waves that knock you over it's the big waves! So everyone who thought light is just a wave was really confused when the intensity of the light was increased (brighter light) and the kinetic energy of the emitted electron did not change. What happens is that as you make the light brighter more electrons are emitted but all have the same kinetic energy.
Well, they thought the kinetic energy of the emitted electron must depend on something. So they varied the frequency of the light and this changed the kinetic energy of the emitted electron.

However, there is a critical frequency for each metal, ν0, below which no electrons are emitted. This tells us that the kinetic energy is equal to the frequency of the light times a constant (i.e., the slope of the line). That constant is called Planck's Constant and is given the symbol h.
h = 6.63 × 10-34 J · s ← Planck's Constant
Now we can write an equation for the kinetic energy of the emitted electron.
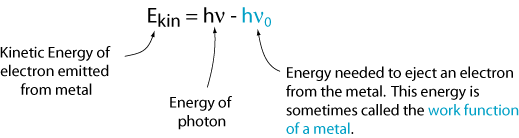
This result is not consistent with the picture of light as a wave. An explanation that is consistent with this picture is that light comes in discrete packages, called photons, and each photon must have enough energy to eject a single electron. Otherwise, nothing happens. So, the energy of a single photon is:
Ephoton = h ν
When this was first understood, it was a very startling result. It was Albert Einstein who first explained thephotoelectric effect and he received the Nobel Prize in Physics for this work.
So, in summary-light is a particle with wave-like behavior.
Homework from Chemisty, The Central Science, 10th Ed.
6.5, 6.8, 6.9, 6.11, 6.13, 6.15, 6.17, 6.19, 6.21, 6.23, 6.25, 6.27, 6.29

